Low risk, Medium risk, High risk Compounding
Table of Contents
Factors influencing risk level include the type of ingredients used (sterile vs. non-sterile), the complexity and duration of the compounding process, and the environment in which compounding occurs. Assigning a risk level helps determine appropriate storage conditions, beyond-use dates (BUDs), and required testing, ultimately protecting patients from contamination and infection risks.
Low Risk Compounding
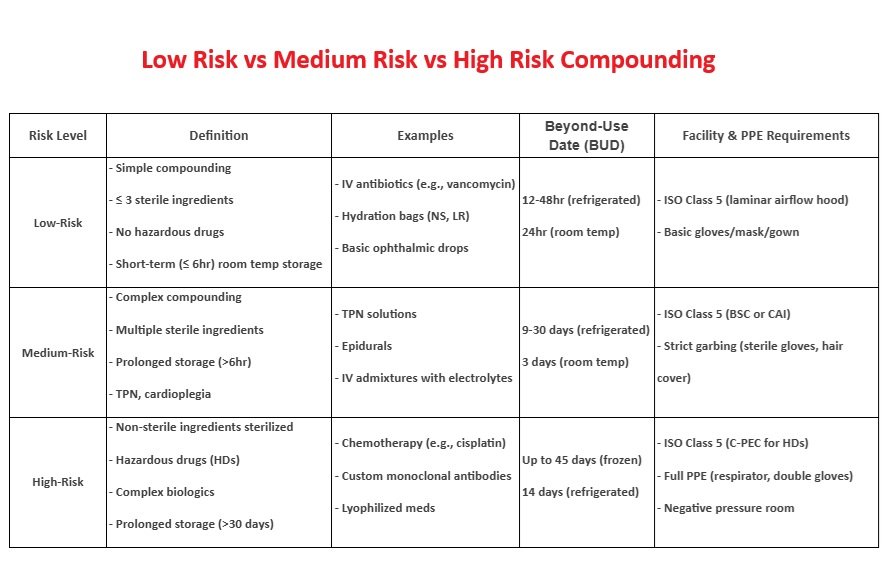
Low risk compounding refers to the preparation of sterile products using aseptic technique with no more than three sterile ingredients, under ISO Class 5 conditions within a cleanroom. It involves simple transfers, mixing, or reconstitution using sterile devices, and poses minimal risk of microbial contamination when properly performed.Definition:
- Prepared in ISO Class 5 environment (e.g., laminar airflow hood).
- Uses sterile ingredients (commercially available, FDA-approved).
- No more than 3 sterile components mixed.
- Simple transfers (e.g., reconstituting a single vial).
Compounding Criteria:
- Simple mixing or transferring of ≤3 sterile ingredients.
- Performed in ISO 5 hood in ISO 7 cleanroom.
- Closed or sealed containers
Low risk compounding examples
Reconstituting a single-dose antibiotic vial:
- Adding 10 mL sterile water to a ceftriaxone 1g vial.
- Why this is low-risk?: Only 2 sterile components (powder + diluent), no complex steps.
Preparing a saline IV flush:
- Drawing up 10 mL 0.9% sodium chloride from a sterile bag into a syringe.
- Why low-risk?: Single sterile component, no additives.
Mixing a premixed IV antibiotic:
- Transferring vancomycin 1g in 100 mL D5W from a manufacturer’s bag to an IV line.
- Why low-risk?: Commercially sterile product, no manipulation.
BUD (Beyond Use Date):
- 48 hours at room temp (or 14 days refrigerated).
Medium Risk Compounding
Medium risk compounding involves sterile compounding procedures that are more complex or prolonged than low-risk, using multiple sterile ingredients or doses for multiple patients. It includes processes like batch preparations or total parenteral nutrition, where aseptic technique is maintained, but the increased complexity raises the risk of contamination.Definition:
- Involves multiple sterile ingredients (4+ components).
- Requires complex manipulations (e.g., pooling sterile products).
- Risk of contamination is higher due to extended compounding time.
Medium risk compounding Criteria:
- Multiple doses of sterile products combined for multiple patients or one patient over multiple doses.
- More than 3 ingredients.
- Requires longer compounding or mixing time.
Medium risk compounding examples
TPN (Total Parenteral Nutrition) preparation:
- Combining dextrose 20%, amino acids 10%, lipids 10%, electrolytes, and vitamins in one bag.
- Why medium-risk?: ≥4 sterile components, prolonged compounding time.
IV chemotherapy admixture:
- Mixing cisplatin 50mg + 500 mL NS + antiemetic (ondansetron).
- Why medium-risk?: Hazardous drug handling + multiple sterile ingredients.
Multi-drug IV piggyback:
- Adding cefepime 2g + metronidazole 500mg to the same 100 mL NS bag.
- Why medium-risk?: Multiple sterile drugs combined.
BUD (Beyond Use Date)
- 30 hours at room temp (or 9 days refrigerated) if sterility testing is performed.
- 24 hours at room temp (or 3 days refrigerated) without sterility testing.
High Risk Compounding
High risk compounding refers to the preparation of sterile products using non-sterile ingredients or devices, or when sterile ingredients are exposed to less than ISO Class 5 air quality. This type of compounding requires terminal sterilization and poses a higher risk of microbial contamination if proper procedures are not followed.Definition:
- Uses nonsterile ingredients (e.g., raw powders).
- Requires sterilization (e.g., filtration, autoclaving).
- High contamination risk (e.g., compounding from bulk chemicals).
High risk compounding Criteria:
- Using non-sterile bulk powders or water for injection.
- Compounding outside of ISO 5 environment.
- Must be terminally sterilized if possible.
High risk compounding examples
Compounding ophthalmic drops from bulk powder:
- Dissolving nonsterile ketorolac powder in sterile water, then filtering (0.22µm).
- Why high-risk?: Starts with nonsterile API, requires sterilization.
Hormone injection from raw materials:
- Creating testosterone propionate injection using bulk powder + oil vehicle.
- Why high-risk?: Nonsterile ingredients, terminal sterilization needed.
Custom IV pain solution:
- Mixing nonsterile ketamine powder + sterile NS, then filtering.
- Why high-risk?: Raw chemical used, must be sterilized before use.
BUD (Beyond Use Date)
- 24 hours at room temp (or 3 days refrigerated)
- unless sterility testing is done (then up to 30 days)
| Risk Level | Components | Manipulations | BUD (Room Temp) |
| Low | ≤3 sterile ingredients | Simple transfers | 48 hours |
| Medium | ≥4 sterile ingredients | Complex mixing | 24–30 hours |
| High | Nonsterile ingredients | Requires sterilization | 24 hours |
Questions and Answers
Question 1: A pharmacy technician is preparing multiple bags of TPN (total parenteral nutrition) solutions for the NICU using sterile ingredients. Each TPN bag requires over 10 separate injections of nutrients and electrolytes into the base solution, performed under ISO Class 5 conditions.
What is the risk level of this compounding activity?
A. Low Risk
B. Medium Risk
C. High Risk
D. Immediate Use
Explanation:
Compounding involving multiple sterile products with complex aseptic manipulations or more than three drug transfers under ISO Class 5 conditions qualifies as medium-risk.
Question 2: A pharmacist asks you how long a medium-risk sterile compounded IV solution can be stored in the refrigerator if it was prepared today under proper aseptic conditions.
What is the maximum beyond-use date (BUD) for medium-risk CSPs stored under refrigeration?
A. 24 hours
B. 48 hours
C. 7 days
D. 9 days
Explanation:
USP <797> states that medium-risk CSPs have a BUD of 30 hours at room temperature, 9 days refrigerated, and 45 days if frozen.
Question 3: A pharmacy technician is asked to prepare an IV solution by withdrawing a dose from a single-dose vial of ceftriaxone and injecting it into a 100 mL bag of 0.9% sodium chloride. The procedure is done in an ISO Class 5 laminar airflow hood located in a clean room.
What is the appropriate risk level of this compounding activity?
A. Low Risk
B. Medium Risk
C. High Risk
D. Immediate Use
Explanation:
This involves transferring sterile drugs in an ISO Class 5 environment using no more than 3 sterile products, which meets criteria for low-risk compounding.
Question 4: A sterile product was prepared under low-risk conditions and stored at room temperature. The pharmacist asks if it is still safe to use after 3 days.
What is the maximum beyond-use date (BUD) for a low-risk compounded sterile product stored at room temperature?
A. 24 hours
B. 48 hours
C. 72 hours
D. 7 days
Explanation:
According to USP <797>, low-risk compounded sterile preparations (CSPs) have a BUD of 48 hours at room temperature, 14 days refrigerated, and 45 days if frozen.
Question 5: A technician is reconstituting a powdered medication that is not sterile and plans to sterilize the final solution using a 0.22-micron filter. All steps are performed under ISO Class 5 conditions.
What is the risk level of this compounding activity?
A. Low Risk
B. Medium Risk
C. High Risk
D. Immediate Use
Explanation:
High-risk compounding includes preparations using non-sterile ingredients or non-sterile devices. Even if sterilized later, the process starts with non-sterile materials, elevating it to high risk.
Question 6: A pharmacist discovers that a technician prepared an IV solution in a clean room where the ISO Class 5 hood was not certified for over 8 months. The solution used sterile ingredients.
Why is this considered high-risk compounding?
A. The IV solution was made using too many vials
B. The technician did not use gloves
C. The environment was not properly maintained
D. The solution was not labeled
Explanation:
Even with sterile components, if the cleanroom or laminar airflow hood is not properly certified or maintained, the risk of contamination increases, making it high-risk compounding.
Question 7: A technician is preparing a low-risk sterile product and is unsure whether they can use a non-sterile powder if it’s mixed aseptically under a laminar airflow hood.
Can non-sterile ingredients be used in low-risk sterile compounding?
A. Yes, if prepared in ISO Class 5 environment
B. No, non-sterile ingredients are not permitted
C. Yes, if stored at refrigerator temperature
D. Only in immediate-use preparations
Explanation:
Low-risk compounding must use only sterile components and equipment; using non-sterile ingredients elevates the compounding level to high-risk.
Question 8: A technician is compounding a batch of antibiotic IVs using 5 different sterile vials and preparing 20 syringes for use over the next few days. All work is completed inside a certified laminar airflow workbench.
Why is this considered medium-risk instead of low-risk?
A. Non-sterile ingredients are used
B. Batch preparation and multiple transfers increase risk
C. Product is exposed to room air
D. It’s performed outside an ISO Class 5 environment
Explanation:
Medium-risk compounding involves batch preparation and complex aseptic techniques, even though all components are sterile and prepared in ISO Class 5. This distinguishes it from low-risk compounding.
Question 9: A pharmacy technician compounds a CSP using non-sterile bulk powder dissolved in sterile water for injection. The final solution is stored at room temperature.
What is the maximum beyond-use date (BUD) for this high-risk CSP stored at room temperature?
A. 24 hours
B. 48 hours
C. 72 hours
D. 14 days
Explanation:
According to USP <797>, high-risk CSPs have a BUD of 24 hours at room temperature, 3 days if refrigerated, and 45 days if frozen, due to the greater risk of microbial contamination.
Question 10: A hospital pharmacy technician is assigned to prepare an intravenous solution of a chemotherapy agent from a non-sterile active pharmaceutical ingredient (API) in powder form.
The technician uses sterile diluent, mixes the solution in a non-certified ISO Class 5 hood within a cleanroom that recently failed environmental monitoring, and plans to terminally sterilize the final product using autoclaving.
The compounded sterile preparation (CSP) will be stored at room temperature and administered 3 days later.
What are the major compounding concerns in this scenario, and what is the appropriate risk level and BUD for this preparation?
A. This is medium-risk due to batch size; BUD is 48 hours
B. This is high-risk due to non-sterile ingredients and failed environment; BUD is 24 hours
C. This is low-risk due to proper sterilization; BUD is 14 days
D. This is immediate-use and should be discarded after 1 hour
Explanation:
This is a classic high-risk compounding situation. Using non-sterile powder, a failed ISO 5 environment, and post-sterilization processing places this in the high-risk category.
According to USP <797>, high-risk CSPs stored at room temperature have a BUD of 24 hours to minimize microbial contamination risks.
Question 11: A technician prepares a batch of preservative-free morphine sulfate epidural solutions using sterile water and morphine sulfate USP powder (non-sterile).
The procedure is carried out in a properly certified ISO Class 5 biological safety cabinet (BSC) located in a negative pressure cleanroom.
The technician uses sterile tools and filters the final product through a 0.22-micron filter but does not perform a sterility test before distributing the product to the anesthesia department for use the following day.
Which of the following best describes the safety issue and compliance problem with this CSP?
A. Use of negative pressure cleanroom requires an immediate-use label
B. Lack of sterility testing is a violation in high-risk batch compounding
C. Use of sterile tools eliminates the need for filtration
D. The use of preservative-free components lowers the risk level
Explanation:
When preparing high-risk CSPs, especially in batch quantities or using non-sterile ingredients, USP <797> requires sterility testing before use if the CSP is not administered within the stated BUD.
Not testing for sterility while preparing a high-risk, injectable product (like an epidural) represents a serious safety and compliance breach.


